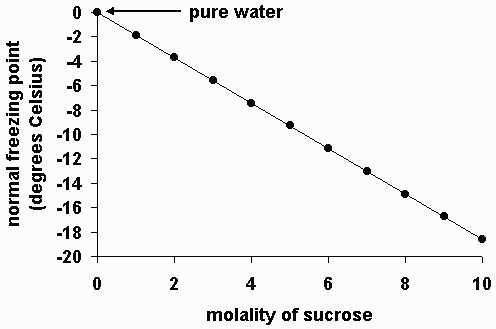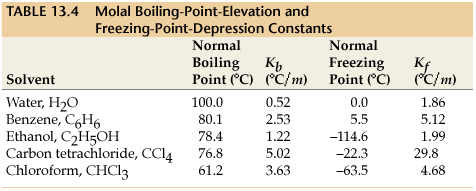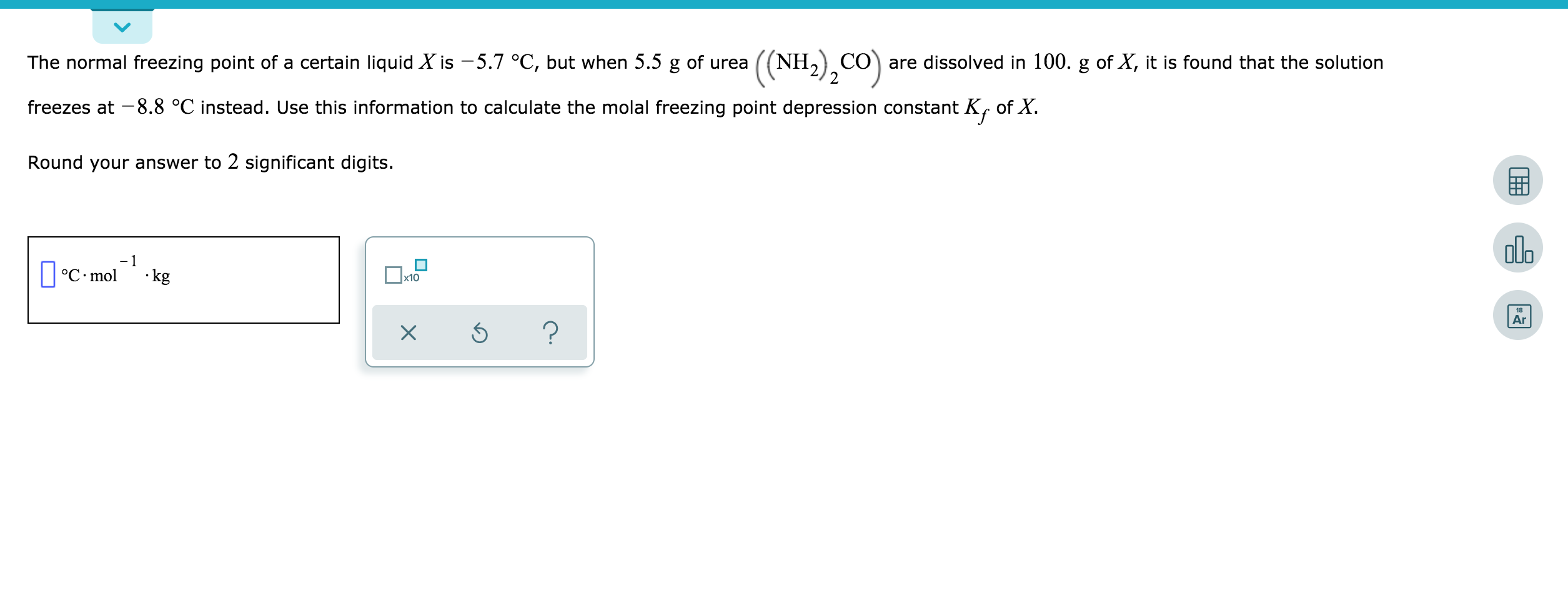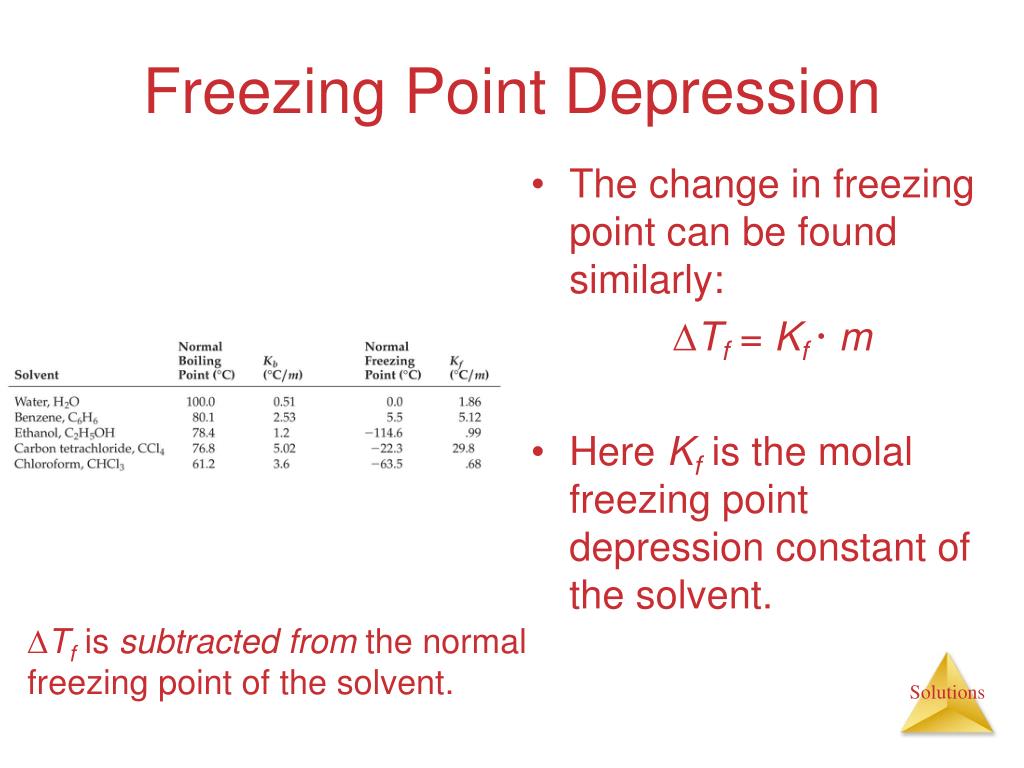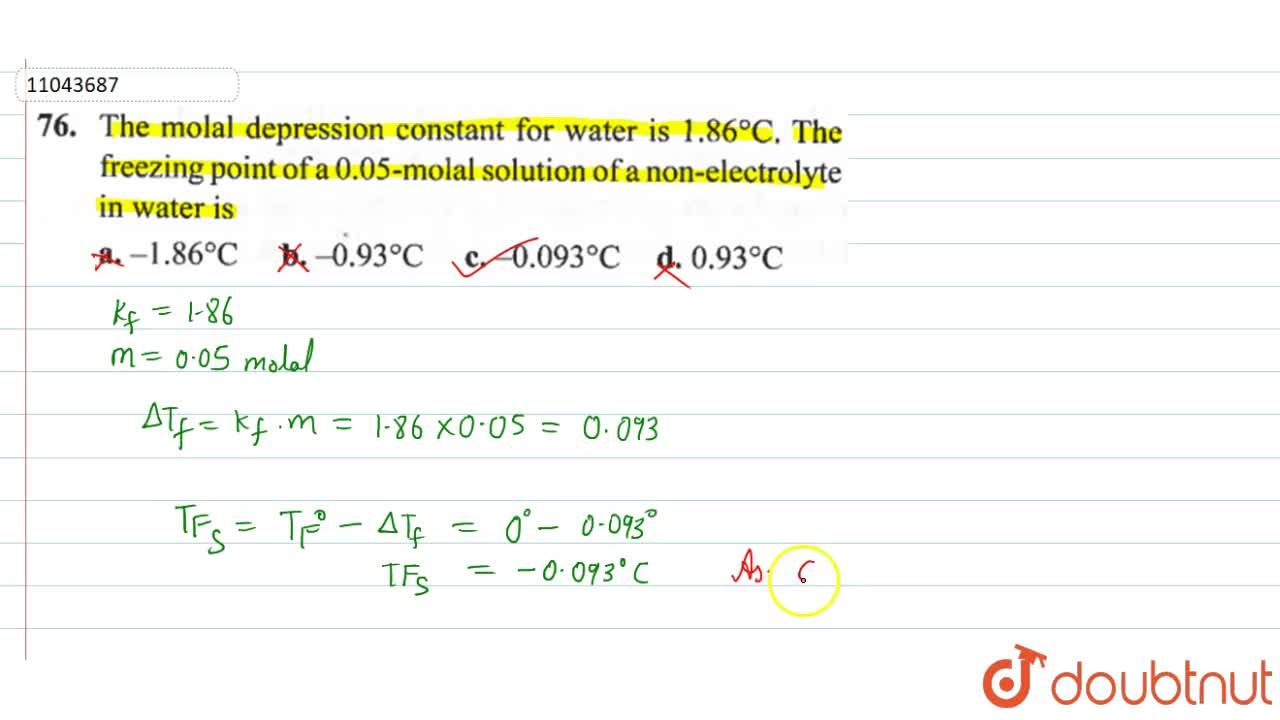
The molal depression constant for water is 1.86^(@)C. The freezing point of a 0.05-molal solution of a non-electrolyte in water is

The molal depression constant for water is `1.86^()C`. The freezing point of a `0.05-molal` solu... - YouTube
The molal depression constant for water is 1.86. The depression constant for 100g is a)1.86b)18.6c)0.186d)186Correct answer is option 'A'. Can you explain this answer? | EduRev JEE Question
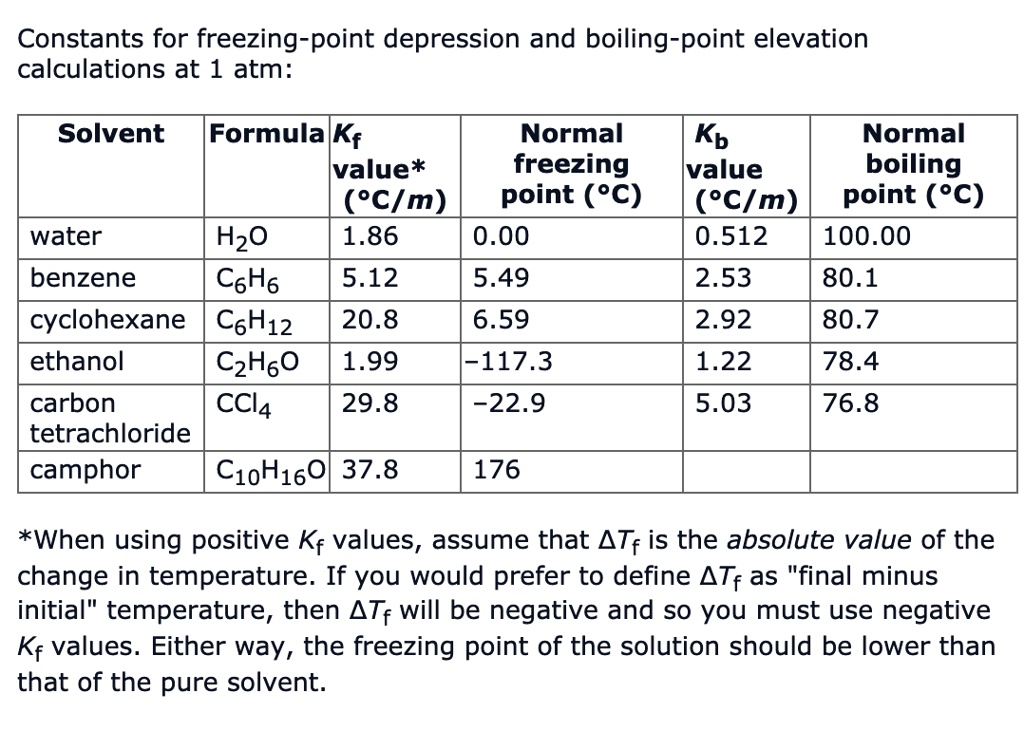
SOLVED: Constants for freezing-point depression and boiling-point elevation calculations at 1 atm: Solvent Formula Kf Normal value* freezing ('CIm) point (%C) water HzO 1.86 0.00 benzene CsH6 5.12 5.49 cyclohexane C6H12 20.8

SOLVED: Data Sheet 2 The Determination of Kr of Naphthalene mass of test tube naphthalene, g 1ul431 mass of test tube naphthalene biphenyl, g 47.440 mass of naphthalene, g T.diSq mass of '

The molal freezing point depression constant of benzene`(C_(6)H_(6))` is `4.90 K kg mol^(-1)`. S... - YouTube

Calculate the molal depression constant of a solvent which has a. Freezing point `16.6^(@)C` and - YouTube