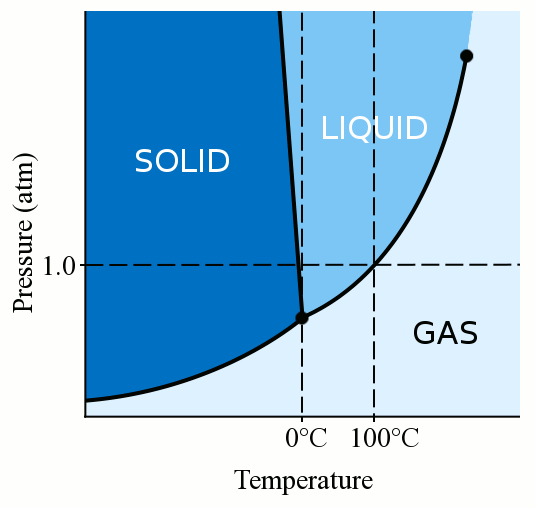
Does salty water freeze at lower degrees than fresh one, or does it just freeze slower than fresh water, but both turns into ice at the 0°? - Quora

General Chemistry Online: FAQ: Solutions: Why isn't 0°F the lowest possible temperature for a salt/ice/water mixture?
How cold would Earth's atmosphere have to be for water at the bottom of the Mariana Trench to freeze? - Quora

Sea Ice Yes it's just frozen sea water.. Freezing Point Fresh water freezes at 0 degrees Celsius (32 degrees Fahrenheit), but the freezing point of sea. - ppt download

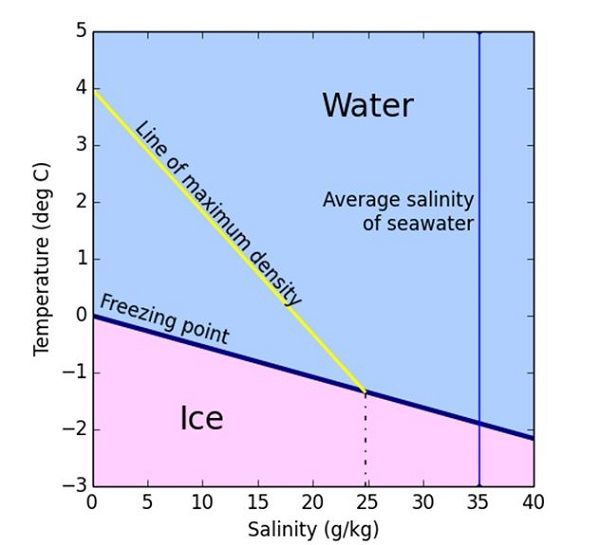
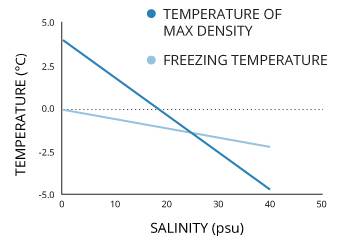
1: Freezing point T f (red, solid) and temperature of maximum density T... | Download Scientific Diagram

Sea Ice Yes it's just frozen sea water.. Freezing Point Fresh water freezes at 0 degrees Celsius (32 degrees Fahrenheit), but the freezing point of sea. - ppt download
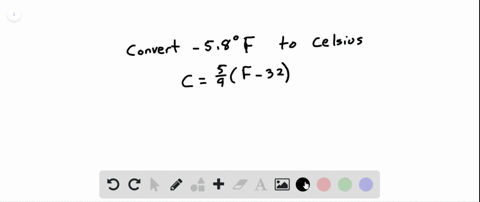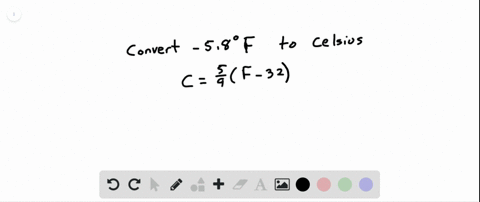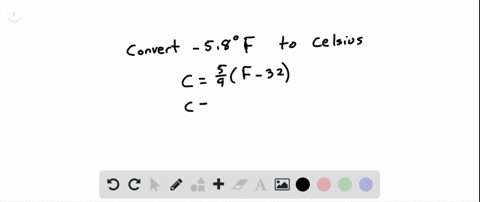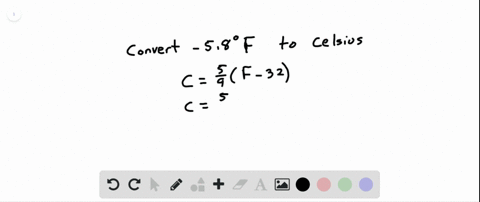
SOLVED:See Example 4. Freezing Points. Saltwater has a much lower freezing point than freshwater does. For saltwater that is saturated as much as it can possibly get (23.3 % salt by weight),

SOLVED: Question 22 3pes Seawater on average has salinity of about 3.5% by mass. Most of that salinity Is due to dissalved sodium chloride: At this concentration the density of the seawater
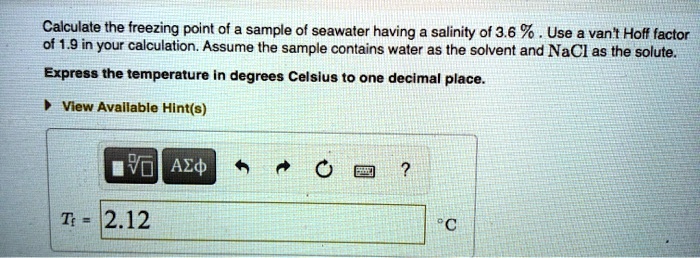
SOLVED: Calculate the freezing point of a sample of seawater having salinity ol 3.6 % Use a van t Holf factor of 1.9 in your calculation. Assume the sample contains water as