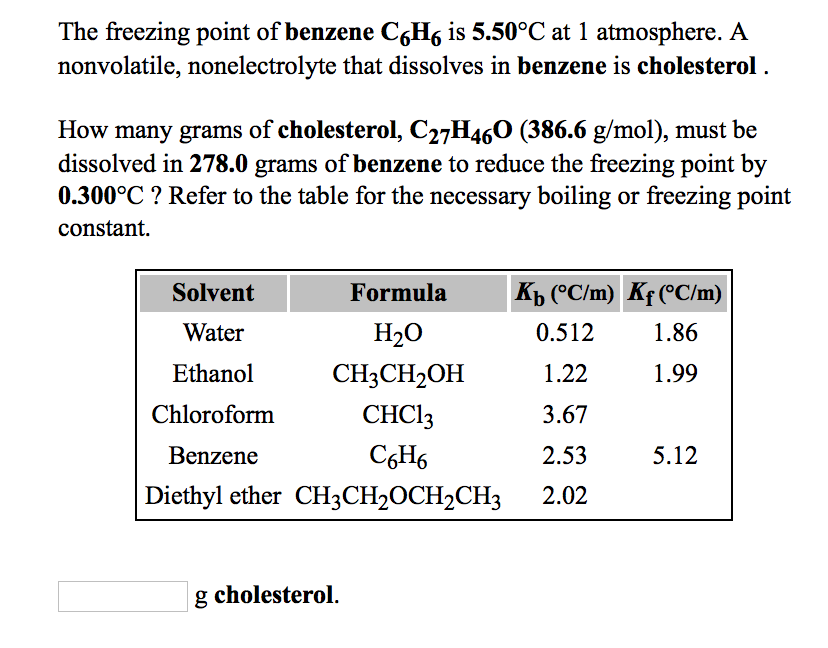
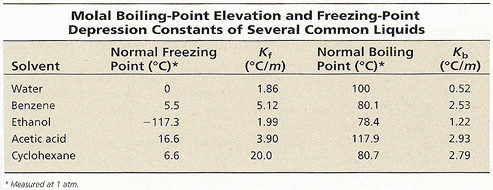
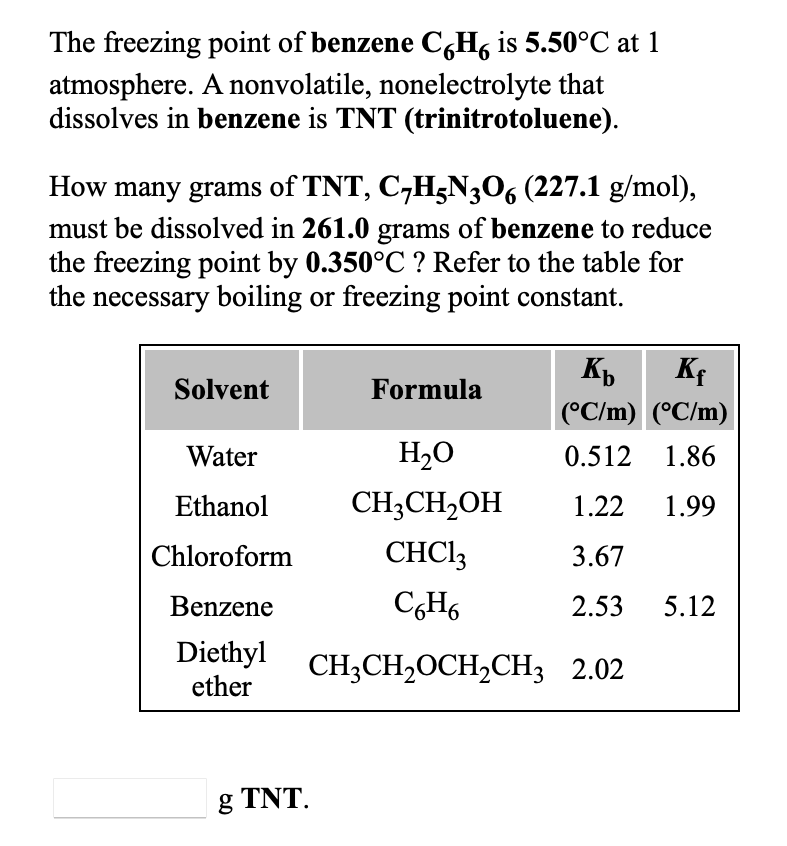
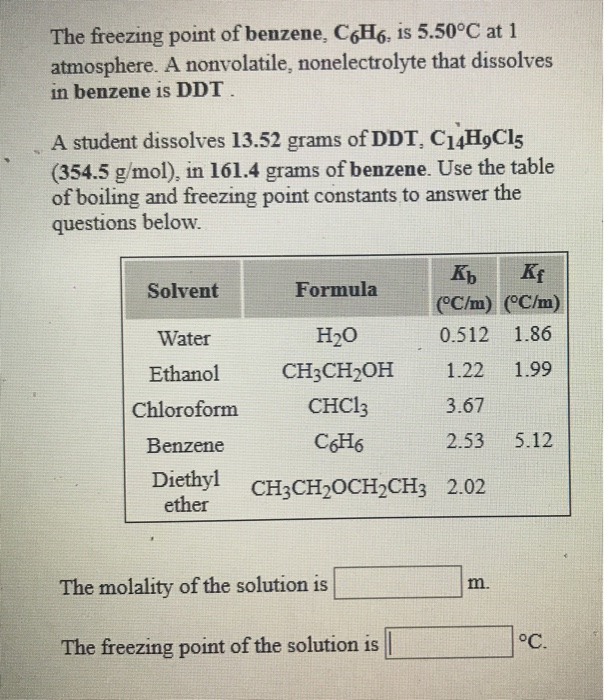
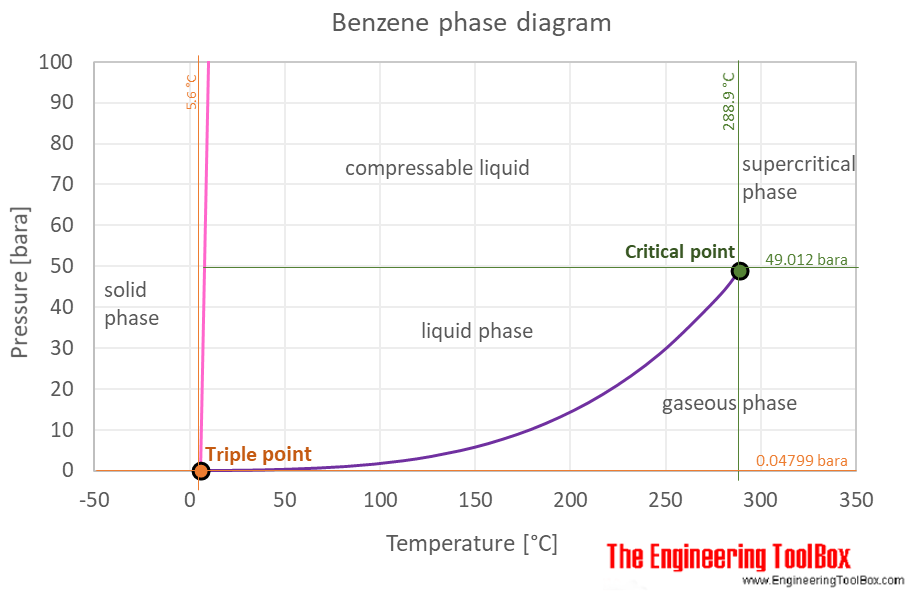
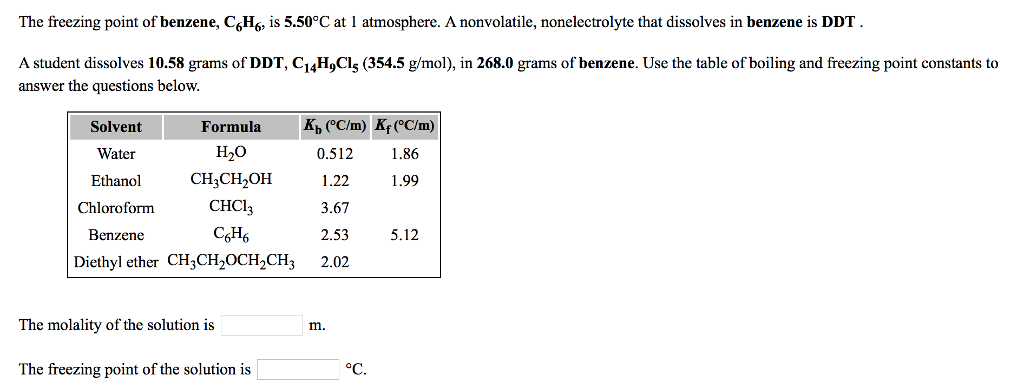
0.15 grams of benzene (C_6H_6) is dissolved into 4.7 grams of melted biphenyl. The freezing point of the mixture was determined to be 4.7 degrees below the freezing point of pure biphenyl.

What happens to freezing point of benzene when nephthalens is added ? | 12 | SOLUTIONS AND COLLI... - YouTube

The molal freezing point depression constant of benzene`(C_(6)H_(6))` is `4.90 K kg mol^(-1)`. S... - YouTube

OneClass: The freezing point of benzene is 5.5 degree C. What is the freezing point of a solution of ...

Benzene melts at `5.50^(@)C` and has a freezing point depression constant of `5.10^(@)C dot" m^(-1)` - YouTube
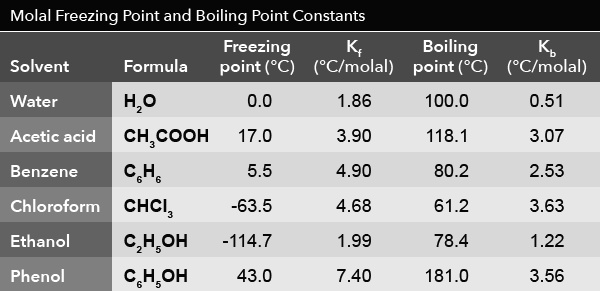
SOLVED: Molal Freezing Point and Boiling Point Constants Freezing Boiling Kb Solvent Formula point (PC) ('Clmolal) point (PC) (Clmolal) Water Hzo 0.0 Acetic acid CH;COOH 17.0 Benzene Cehs 5.5 Chloroform CHCI -63.5
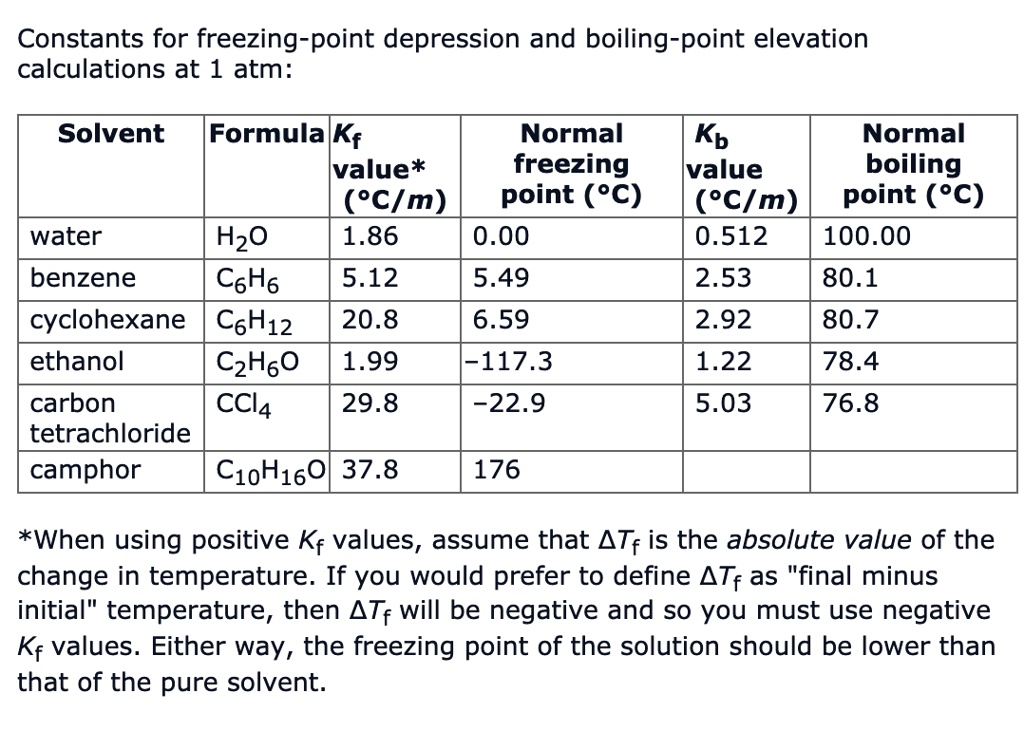
SOLVED: Constants for freezing-point depression and boiling-point elevation calculations at 1 atm: Solvent Formula Kf Normal value* freezing ('CIm) point (%C) water HzO 1.86 0.00 benzene CsH6 5.12 5.49 cyclohexane C6H12 20.8
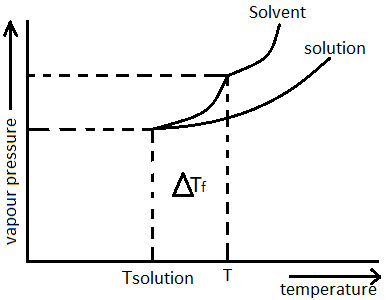
The freezing point of benzene decreases by ${0.45^0}C$ when $0.2$ g acetic acid is added to $20$g of benzene. If acetic acid associates to form a dimer in benzene, percentage association of
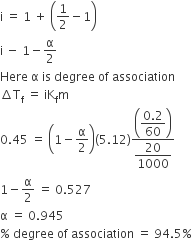
The freezing point of benzene decreases by 0.45°C when 0.2 g of acetic acid is added to 20 g of benzene. If acetic acid associates to form a dimer in benzene, percentage

What is the (a) molality, (b) freezing point, and (c) boiling point of a solution containing 2.29 g napthalene (C_{10}H_8) in 44.6 g of benzene (C_6H_6)? | Homework.Study.com

The freezing point of benzene decreases by 045°C when 0.2 g of acetic acid is added to 20 g of benzene

The freezing point of benzene decreases by 0.45^0C when 0.2 g of acetic acid is added to 20 g of benzene. If acetic acid is associates to form a dimer in benzene,