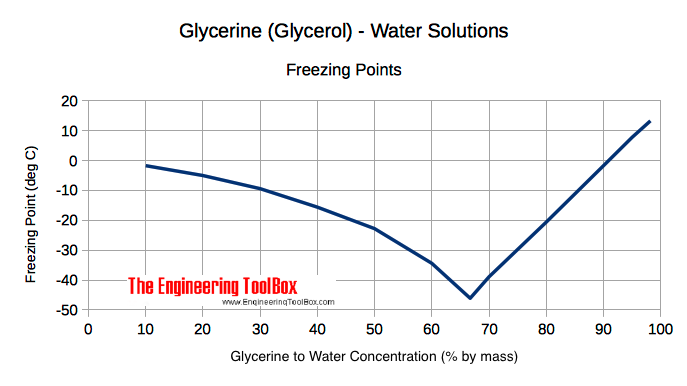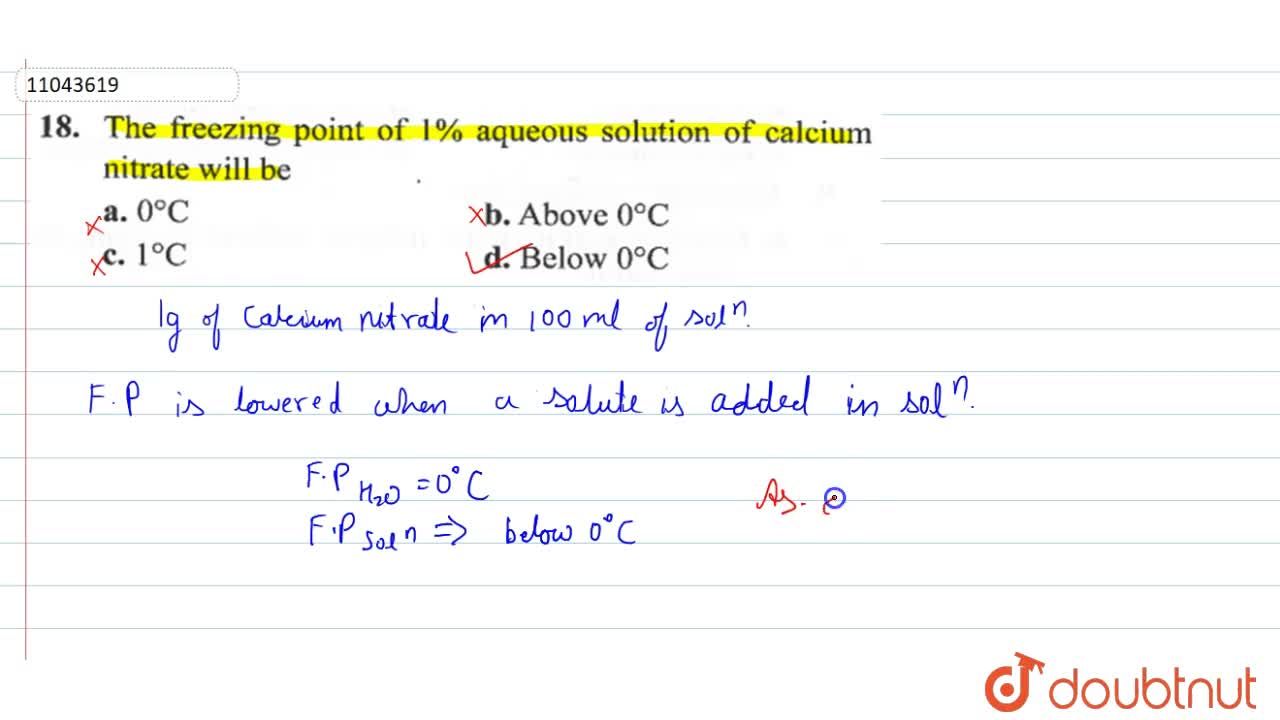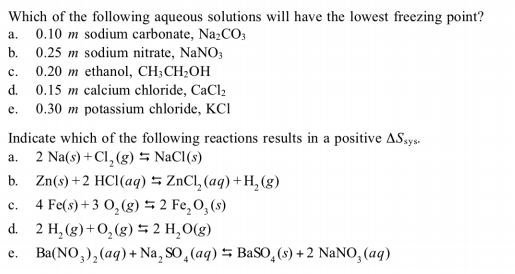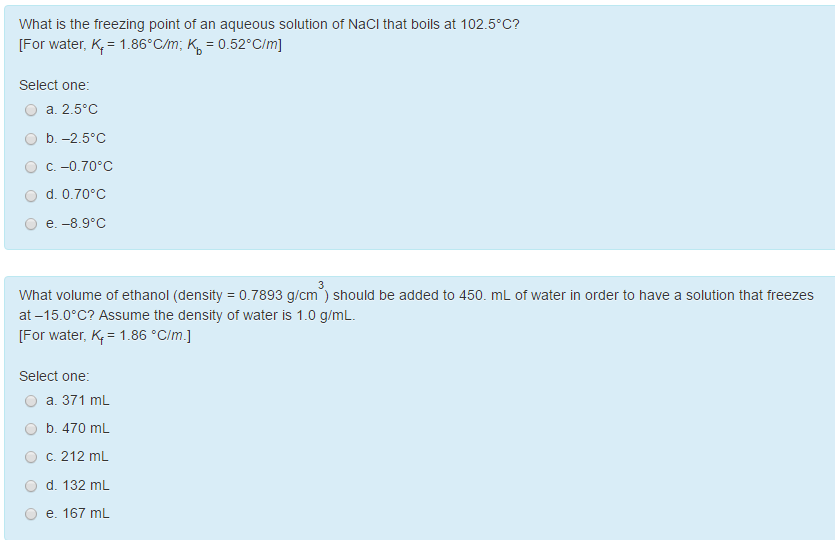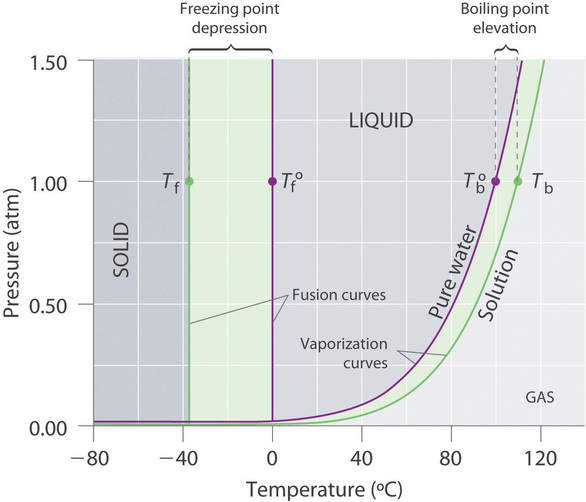
13.8: Freezing-Point Depression and Boiling-Point Elevation of Nonelectrolyte Solutions - Chemistry LibreTexts
What is the freezing point of an aqueous solution containing 10.50 of MgBr2 in 200g of water? - Quora

Freezing point of a 4% aqueous solution of X is equal to freezing point of 12% aqueous solution of Y . If molecular weight of X is A , then molecular weight of Y is:
The Freezing Points of Aqueous Solutions. X. Dioxane and its Mixtures with Lithium, Sodium and Potassium Chlorides1 | Journal of the American Chemical Society
What is the freezing point of an aqueous solution containing 10.50 of MgBr2 in 200g of water? - Quora

What should be the freezing point of aqueous solution containing 17g of C(2)H(5)OH is 1000g of water (K(f) for water =1.86 deg kg mol^(-1))?
9. Calculate the freezing point of an aqueous solution of non electrolyte having osmotic pressure of 2.0 atm at 300K. (Kf = 1.86 kg/mol , R = 0.0821 L atm/ K mol )

Calculate the freezing point of an aqueous solution containing `10.5g` of Magnesium bromide in - YouTube
![The freezing point of an aqueous solution of a non - electrolyte is - 0.14^∘C . The molality of this solution is : [kf (H2O) = 1.86 K kg mol^-1] The freezing point of an aqueous solution of a non - electrolyte is - 0.14^∘C . The molality of this solution is : [kf (H2O) = 1.86 K kg mol^-1]](https://dwes9vv9u0550.cloudfront.net/images/964943/64b4be52-3ed8-4f23-ac85-76b844a2fb98.jpg)