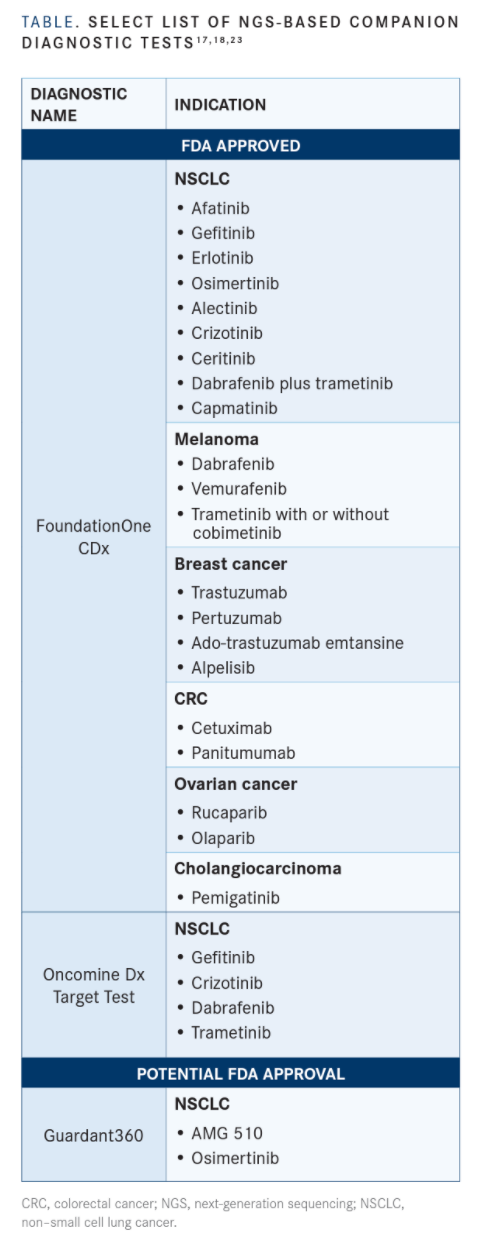
Foundation Medicine Receives FDA Approval for FoundationOne®Liquid CDx as a Companion Diagnostic for a Certain Group of Tyrosine Kinase Inhibitors for Treatment of Non-Small Cell Lung Cancer Patients | Business Wire
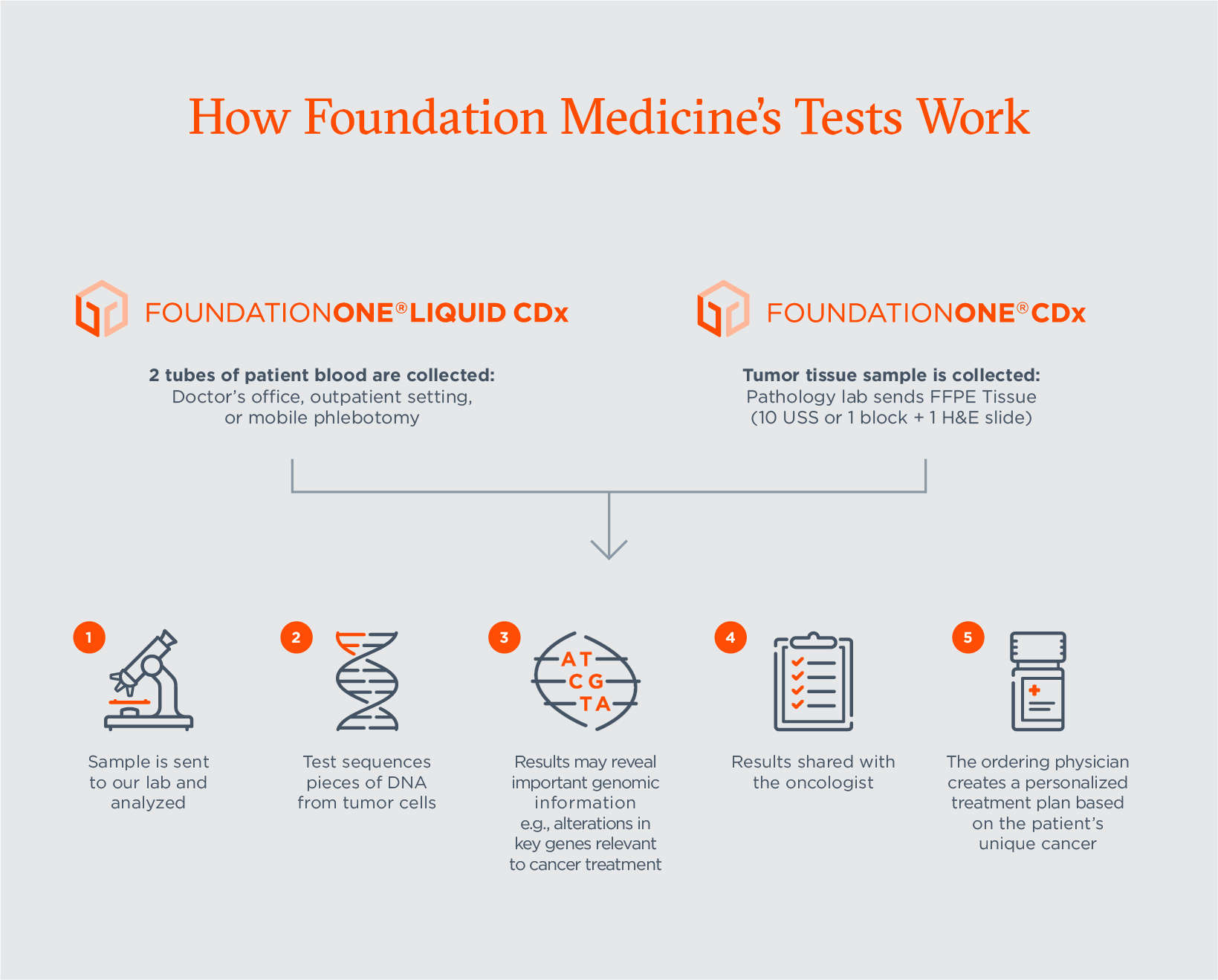
Companion Diagnostics Explained: Their Critical Role in Cancer Care and Our Latest Approvals | Foundation Medicine
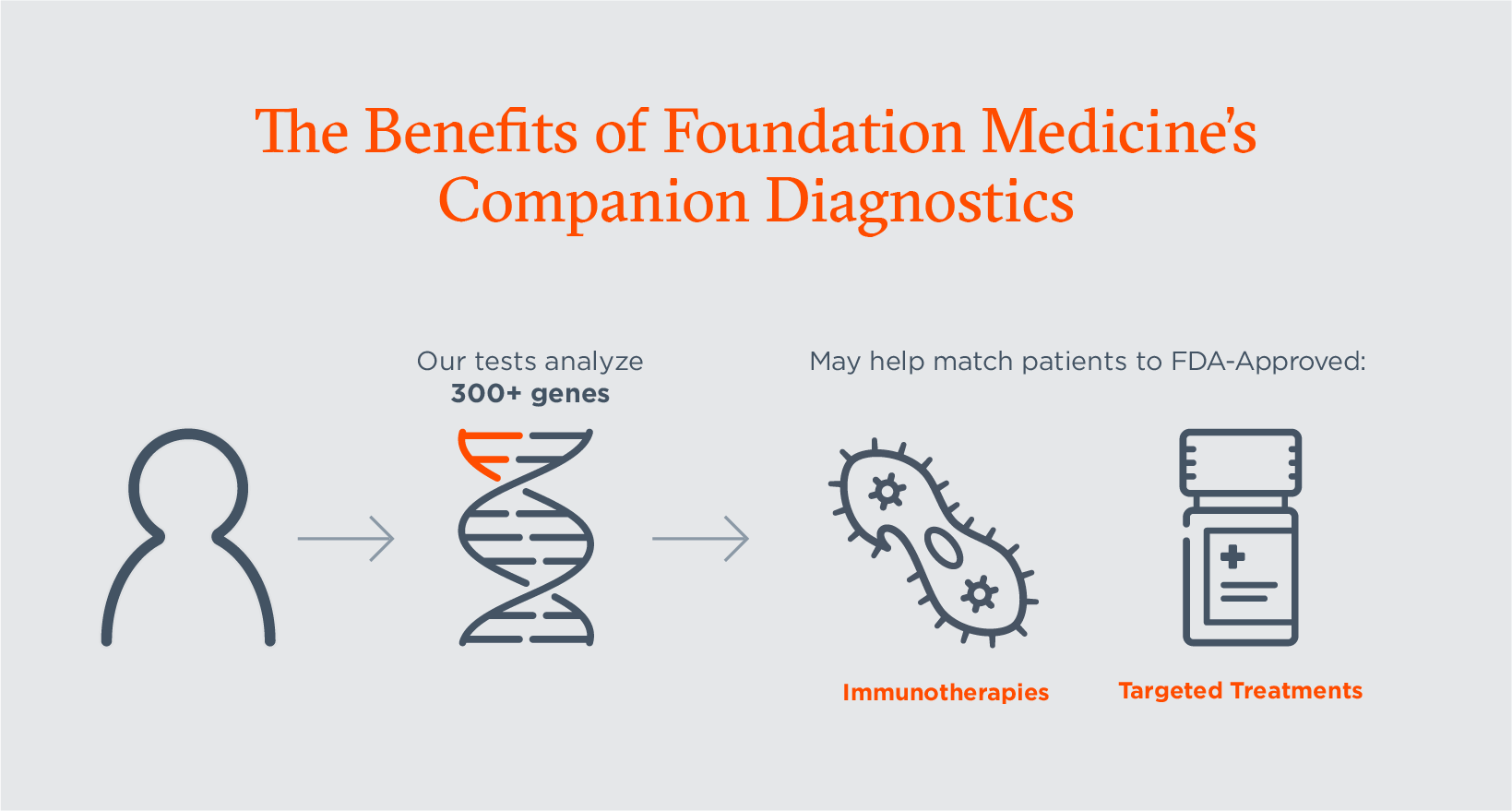
Companion Diagnostics Explained: Their Critical Role in Cancer Care and Our Latest Approvals | Foundation Medicine
Clinical and analytical validation of FoundationOne®CDx, a comprehensive genomic profiling assay for solid tumors | PLOS ONE